The inspirational quotation at the top of Upper School science Preceptor Suzie Jekel’s syllabus for the two-trimester course Advanced Biology: Tiny Earth reads, “Let us crush pesky bacteria before they crush us or try to.”
It’s not exactly a course description, but it does capture accurately the sense of urgency around the type of scientific research that is at the center of this one-of-a-kind, AP-level offering at Colorado Academy.
Headquartered at the University of Wisconsin-Madison, the Tiny Earth initiative arose just over a decade ago to address one of the most pressing global health challenges of the century: the diminishing supply of effective human antibiotics. The benefits of these “miracle” treatments have steadily declined as prolonged use of existing medications such as penicillin in large populations has driven protective mutations in the target bacteria, and as the pharmaceutical industry has shifted away from antibiotic discovery. Current antibiotic-resistant threats range from well-known pathogens such as Streptococcus pneumoniae and Escherichia coli to “superbugs,” including multi-drug-resistant Myobacterium tuberculosis and Methicillin-resistant Staphylococcus aureus (MRSA).

Successful antibiotics, like penicillin, are naturally produced by one microorganism fighting another. Antimicrobial substances that might be discovered in the world’s soil (or in mold, like penicillin) are seen as one of the best hopes for combating emerging, potentially deadly infections. As of today, Tiny Earth’s network of 800+ instructors and more than 16,000 students a year at 540 educational institutions worldwide has turned up tens of thousands of pathogen-inhibiting isolates from local soil samples, with 28 of these identified as bioactive compounds that could show the way toward new antibiotics to combat the resistance crisis.
Most students and teachers who are part of the Tiny Earth network are working in labs on college and university campuses; it is primarily these types of facilities that can support the kind of rigorous, carefully controlled research that is required in antibiotic discovery, which uses real pathogens as test subjects for promising antibiotic candidates. That the program exists at CA, one of the only high schools anywhere in the world to take part, is due to the efforts of one person; Master Teacher Dani Meyers, who retired in 2022 after 27 years in the Upper School Science Department.

Like Jekel, Meyers was drawn to the project because of the urgency surrounding the antibiotic resistance crisis, as well as her own fascination with the human immune system. “It’s wild,” Meyers says of the mechanisms by which the human body fights infections of all kinds. “Before I came to CA, I was focused on immunology and parasitology, and I spent time at medical facilities in Kenya and Uganda doing immunizations. The infections that came through the door there were insane; it’s what made me realize this field is so interesting and important.”
Meyers saw Tiny Earth as a unique opportunity for CA students to participate in practical scientific research with the potential to literally change the world. Not long after the initiative went public at the University of Wisconsin, she was on the phone with Tiny Earth administrators to persuade them that CA teachers and students could make real contributions to the project, and that its facilities and resources were able to support the required safety protocols and lab procedures, including the use of a sophisticated thermal cycler in DNA amplification.
“For me, science is all about hands-on experience,” recounts Meyers. And indeed, it took Meyers some careful fine tuning with one student enrolled in an independent study—Maggi Davis ’18, daughter of Head of School Dr. Mike Davis—to ready the course for inclusion in the Upper School science curriculum. “We ended up melting a few plastic cell culture dishes,” she says, but after a couple of tries, Tiny Earth officially became part of the course catalog in 2019, and responsibility for running the class went to Jekel after Meyers retired.
Serious science
Five years later, this advanced biology course stands as a beacon for Upper School students, whether they are pursuing hard science with an eye to a scientific career or are simply interested in the challenge of hunting for potential cures for illnesses that affect millions around the globe. One very big reason the course has a long waiting list of Juniors and Seniors year after year is because it’s “no joke,” according to Jekel: “This is a very real problem in the world, and in this class we deal with very real relatives of some of the most concerning pathogens out there.”

The course begins with students carefully collecting soil samples near their homes across the Denver Metro Area. Research indicates that one gram of soil contains tens of thousands of bacterial species and billions of individual cells, and as a result, the majority of antibiotics in commercial and clinical use today are derived from soil bacteria. The samples that the students collect are serially diluted to produce a workable number of microbes, and the dilutions are then cultured on plates coated with a medium to encourage the growth of bacteria.
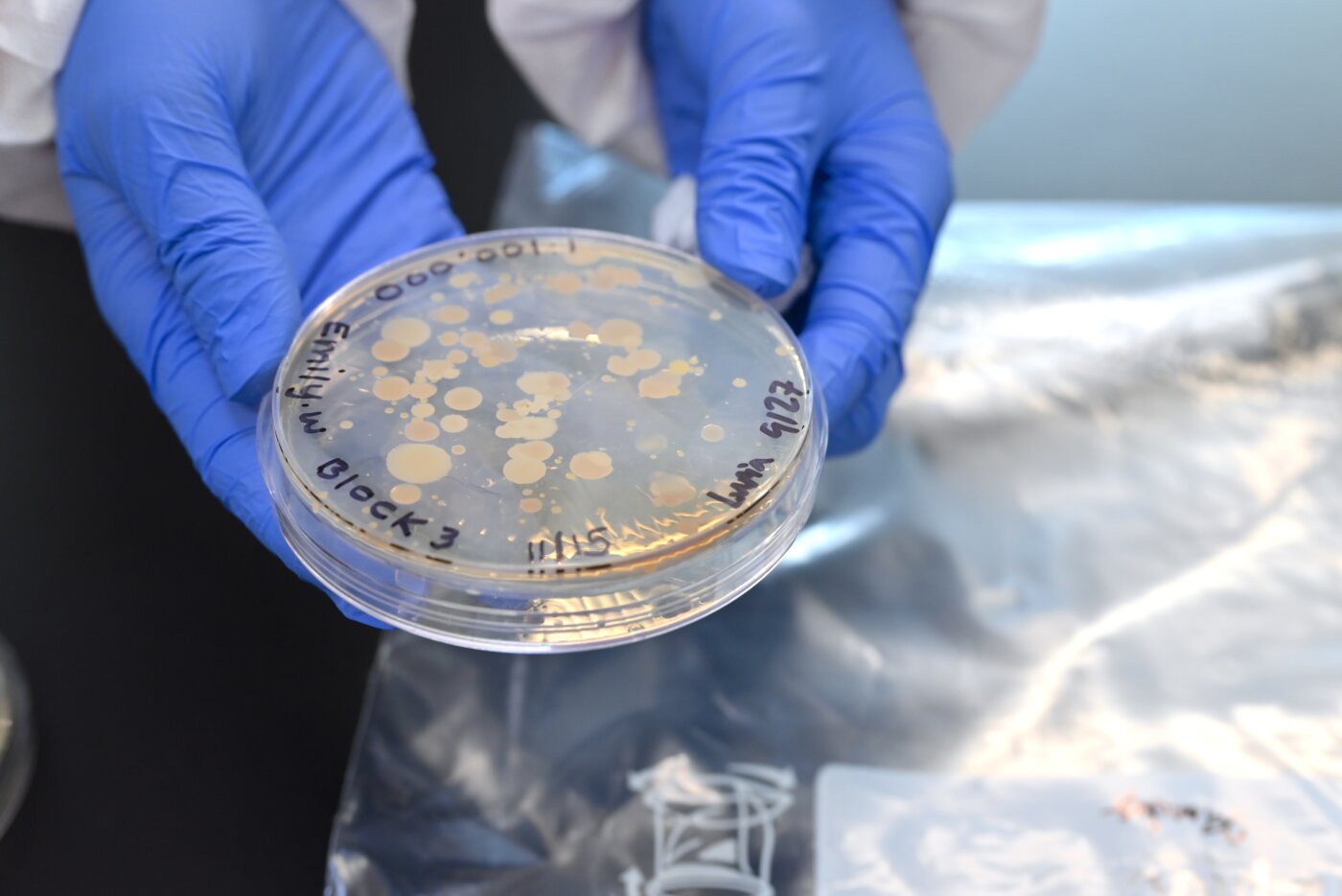
Once students can observe bacterial colonies forming, they apply tiny amounts to “streak” plates to isolate single species that can be further studied for their effectiveness against the pathogenic relatives—named ESKAPE pathogens after the six common infectious organisms they resemble. These comprise the majority of antibiotic-resistant infections seen in health care settings, and so any newly discovered bacteria that can inhibit one or more of them is a good candidate for more research.
Promising bacterial strains stand out on specially prepared “fight” plates, and these are analyzed using the PCR (polymerase chain reaction) method, which amplifies DNA via a thermal cycler to make identification easier. The students’ final results are compared to public antibiotic DNA datasets and submitted to the official Tiny Earth database at the University of Wisconsin, where scientists use the data to continue the search for novel antibiotics.

From start to finish, Jekel observes, this exacting process in CA’s science labs offers students “unmatched experience with the kind of work they’d be doing in college or in a commercial setting. They learn how to make their own solutions, run tests, design an experiment. They understand the importance of aseptic technique—probably the most important part of working in a microbiology lab. My graduates tell me they’re miles ahead of their college peers.”
Almost more significant, Jekel makes clear, is the way that the tedious, seemingly arbitrary nature of antibiotic discovery itself gives students abundant experience with failure.
“Something like 90% of research science consists of failure. All those published journal articles we read? They only come after years and years of failing. Students are initially uncomfortable with that—they want more certainty about the outcome.”

Yet the good news for students in Tiny Earth is that failure doesn’t count against you. On the contrary, it’s at the core of discovery and innovation. “There’s no linear scientific process; it’s not even circular. It involves intuition and iteration: You get some ideas, you do some experiments, and then you look at your data to see what might come out of it. You design more tests and follow a path until you fail again. You’ve got to be willing to get the answers wrong and start over, again and again.”
And in Tiny Earth, Jekel adds, the antibiotic-resistance crisis means the stakes of this quest are real.
People who are part of the CA community, she explains, may not have experience with the frightening infections that are a way of life in other parts of the world; they generally are well fed, have good access to health care, and live in relatively clean environments. “So I give them lots of stories and examples throughout the course that demonstrate that yes, these antibiotic-resistant bacteria are part of our world; the safe ESKAPE relatives we use in class really are infectious, and our aseptic technique is critical. This is a situation where we have to do things right; if we don’t, our health or our career or other people’s lives could be at risk.”
Exploring the next frontier
Senior Noah Keil, who is pursuing an independent-study followup after taking Jekel’s course as a Junior, says of the real-world scientific experience, “Tiny Earth was probably my favorite class ever. And what was so exciting about it was the possibility for genuine scientific discovery in school. I feel like in so many classes, you’re learning and maybe preparing to go change the world, but in Tiny Earth, it’s different. You’re actually doing hands-on science and possibly making a discovery that no one else has.”

In his independent study, which Jekel is supervising, Keil is looking into possibilities at the next frontier of antibiotic research and development: using CRISPR gene-editing technology to target and deactivate genes that cause antibiotic resistance, which can make bacteria more sensitive to antibiotics or kill them. CRISPR can be delivered to bacteria using phages, viruses which are natural predators of bacteria.
Since Seventh Grade, Keil explains, “I’ve just been fascinated with microbiology in general. It’s so small and sophisticated: On the head of a pin you can fit massively complicated systems. And in Tiny Earth in particular, the idea of using bacteria to fight bacteria really appealed to me—you’re turning a system against itself.”

In his final paper for Tiny Earth, Keil wrote about the biggest issue with all antibiotics: the unwinnable “rat race” that starts every time a new treatment is released and bacteria begin evolving resistance to it. Now, on his own, he’s experimenting with CRISPR’s ability to detect specific DNA sequences to see if it might be able to attack bacterial RNA. “This is science that is very current,” notes Keil. “The most recent Nobel Prize in Medicine was given for a new discovery about RNA regulation, and the whole concept of RNA editing is essentially the leading edge of today’s science.”
If Keil’s plans to pursue a biology major in college next year pan out, the preparation he’s gained in Jekel’s classroom will surely help him hit the ground running. Along with antibiotic resistance, the COVID-19 pandemic has sparked a new surge of interest at many institutions in the biology of viruses and microorganisms; the growth of the biotechnology industry, environmental concerns, and the need for new drugs and biofuels are all expected to increase demand for microbiologists in the coming years.
And perhaps Keil or someone like him will help address what Jekel sees as one of the biggest weaknesses in humanity’s fight against bacteria: ignorance.
“When the West Nile virus was first discovered in North America in 1999, I remember a news story that said, ‘Well, here in Colorado, we don’t have to worry about West Nile, because we have such a dry climate,’” Jekel recounts. Colorado has since become one of the U.S. states hardest hit by the mosquito-borne virus, with 2023 seeing 634 cases, 386 hospitalizations, and 51 deaths.

While the disease is caused by a virus rather than bacteria, the parallel with the rise of antibiotic resistance is clear. “We simply need better literacy about the threats to our health that are out there.”
We also need more and better treatments, says Jekel; the “crowdsourcing” model exemplified by Tiny Earth may well be the only way to get there.
